Research
Super-Resolution Ultrasound Imaging
Contrast-Enhanced Angiography
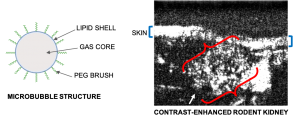
By injecting inert, FDA-approved microbubble contrast agents into the bloodstream, we are able to visualize vasculature that is otherwise difficult to detect with ultrasound.
Image processing techniques of ultrasound localization microscopy allows us to determine precise locations of the microbubbles within the blood vessels.
By accumulating these microbubble localizations over thousands of frames, a super-resolved image of the vasculature is obtained. We call this image ‘super-resolved’ since its ability to distinguish one vessel from another is better than what we would otherwise be able to achieve due to diffraction limitations.
Ultra-High Frame Rate Transcranial Imaging
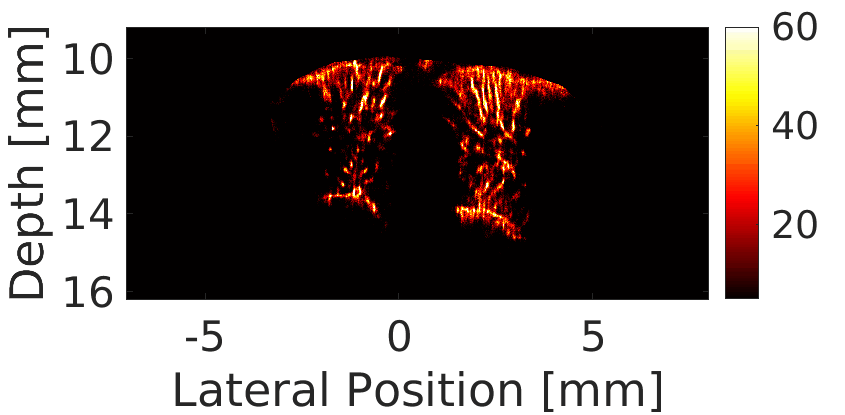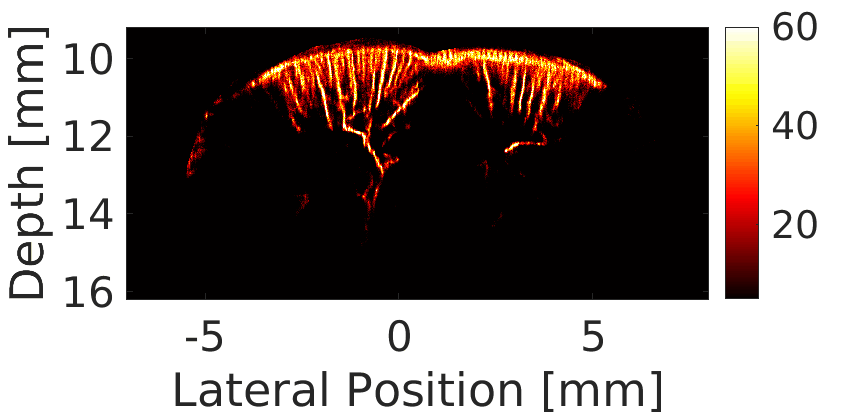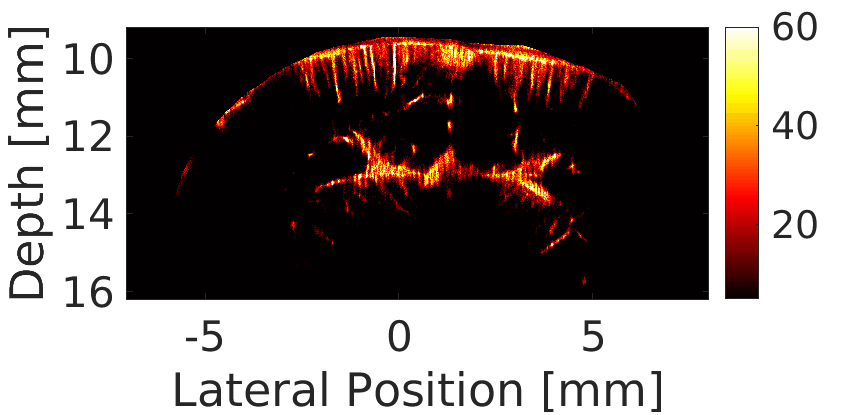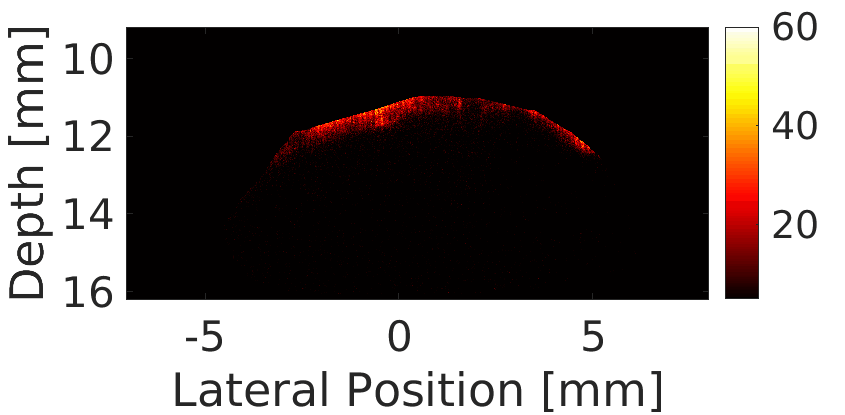
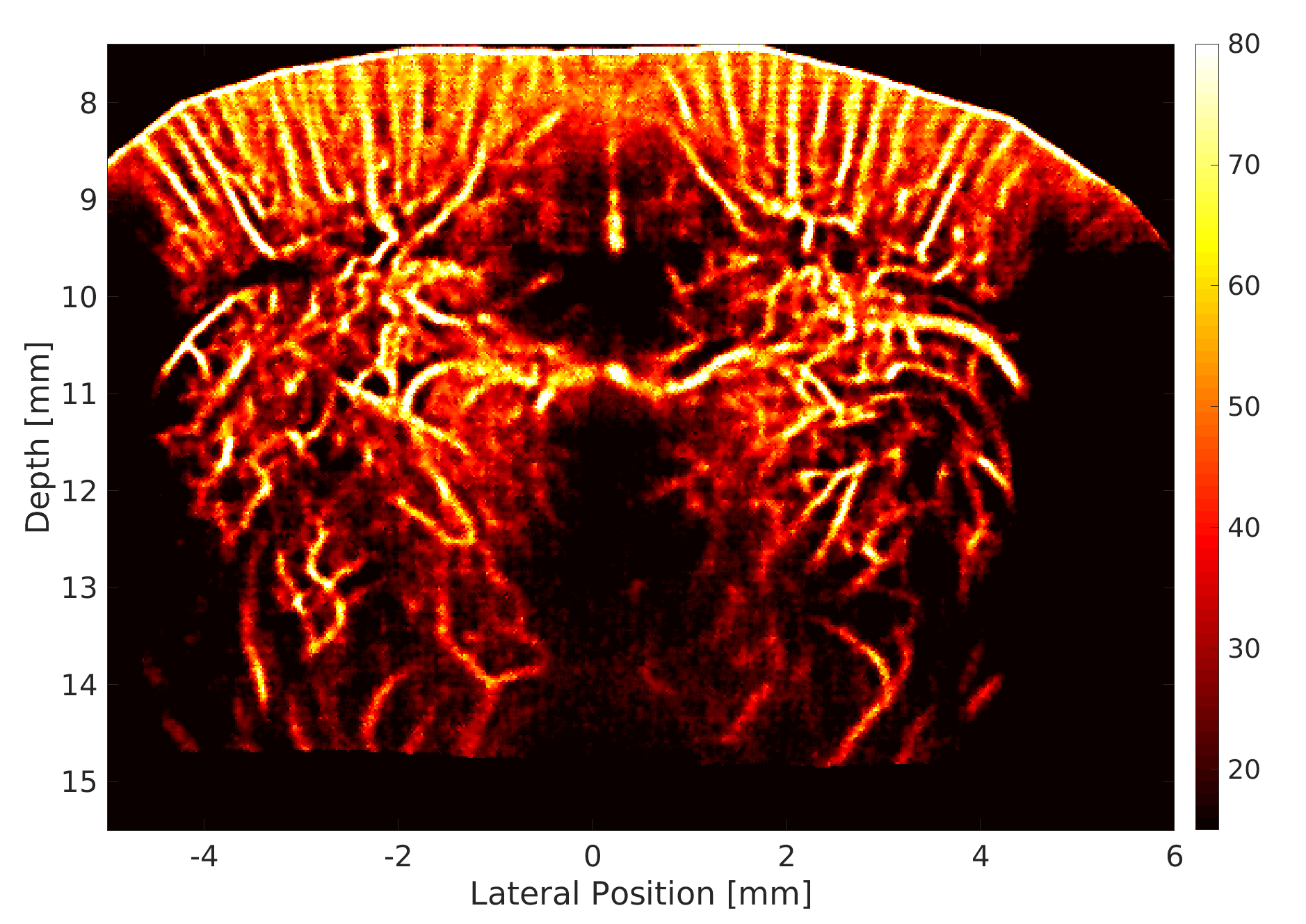
We use a custom build imaging sequence in order to generate a Power Doppler image data set as well as a super-resolved image of in-vivo vasculature, such as in a rat’s brain, shown here.
Our imaging sequence implementation allows to stream in real time from the scanner to the controller’s hard drive, effectively eliminating dead times during the acquisition.
We use this powerful tool for studying the circulatory response dynamics in the brain and the evolution of traumatic brain injury (TBI) in in-vivo animal model.
Transcranial imaging & numerical simulations
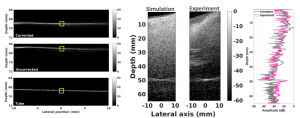
We have demonstrated the feasibility of human transcranial super-resolution imaging through a geometric focusing approach combined with phase correction approach that takes the skull morphology into account.
Fullwave is a numerical solver based on finite differences in the time domain which can be used to model ultrasound propagation in 2D or 3D with a high dynamic range. Experimental conditions can be closely mimicked and predictions on image degradation can be made. Using CT scans of skulls, accurate acoustical maps can be generated and used as inputs in Fullwave.
Neuromodulation
Neuromodulation array for non-human primates using transcranial-focused ultrasound
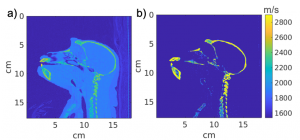
Simulations can be used to design ultrasound arrays based on specific design parameters.
To design neuromodulation arrays for non-human primates, we can convert CT scans (a) of macaque monkeys to acoustic maps (b) which can be used to design and optimize neuromodulation arrays that can focus to specific locations within the brain based on their specific skull morphology.
To perform an action, the brain acts like a circuit and simultaneously recruits neurons from multiple regions within the brain. We have designed a customizable neuromodulation array that can be used to simultaneously stimulate multiple regions inside the brain of non-human primates with an imaging aperture to allow for additional functional imaging.
Shear Waves in Traumatic Brain Injury
Shear shock wave propagation in brain during traumatic impacts
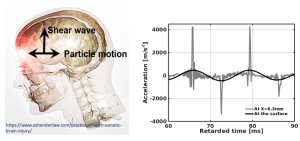 Shock formation in shear mode in the brain may be responsible for diffuse axonal injuries under Traumatic Brain Injury (TBI) impacts.
Shock formation in shear mode in the brain may be responsible for diffuse axonal injuries under Traumatic Brain Injury (TBI) impacts.
Shear waves in tissue transition from smooth to a shock profile in less than one wavelength. Using high frame-rate ultrasound and high sensitivity RF tracking, we observed the formation of shear shock waves with destructive accelerations in the brain.
We observed shear shock waves in the in situ porcine brain during a single-shot impact, by developing a custom interleaved ultrasound sequence with optimized wide-beam emissions.
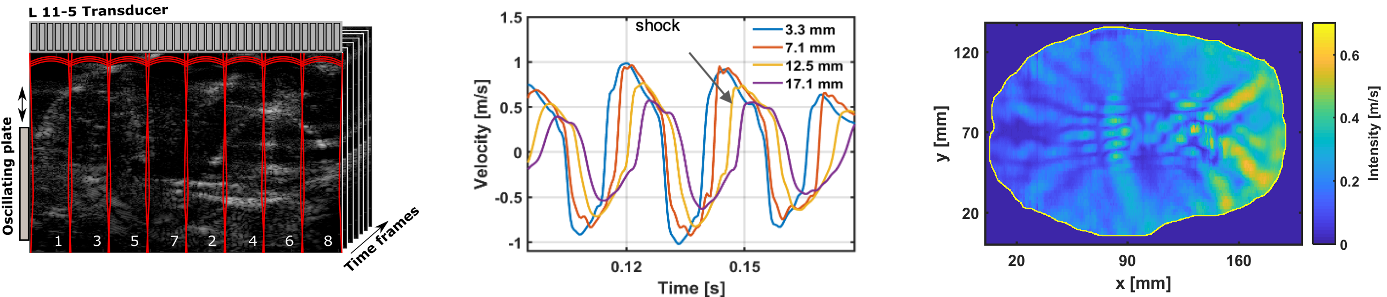
Characterizing shear wave propagation in heterogenous soft solids
Shear wave propagation in brain tissue is heavily implicated in traumatic brain injury but is poorly understood. We use high-frequency ultrasound to characterize the physical properties of shear waves through biological tissue.
Brain tissue is highly variable, so we use modified soft-solid models to investigate how shear wave propagation varies in a heterogeneous environment. Our phantom modifications include liquid-filled voids, periodic gel layers, and other random interspersions.
